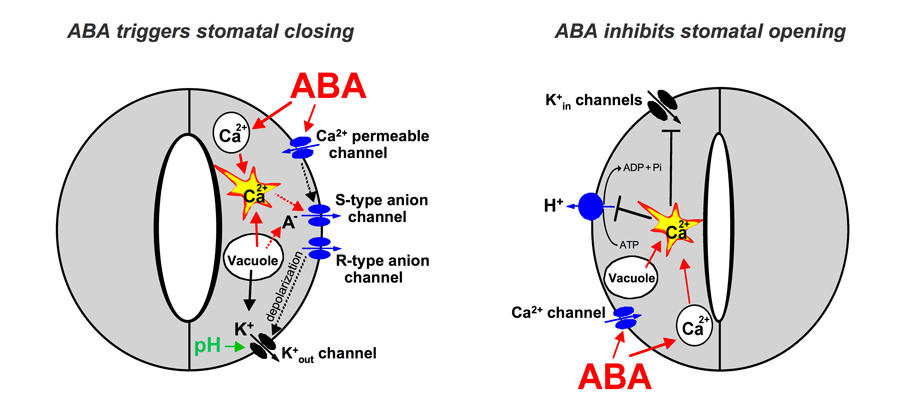
Figure 1: Simplified model for the roles of ion channels and pumps in regulating stomatal movements. Note that other 2nd messengers in addition to Ca2+ also function in stomatal movements (see text for details). Mouse-over for explanations (may not work on all browsers). The hormone ABA triggers a signalling
cascade in guard cells that results in stomatal closure and inhibits stomatal opening. Stomatal closure is mediated by turgor reduction
in guard cells, which is caused by efflux of K+ and anions from guard cells, sucrose removal, and a parallel conversion
of the organic acid malate to osmotically inactive starch (MacRobbie, 1998).
Figure 1 shows an extension of early models for the roles of ion channels in ABA-induced stomatal closing (Schroeder and Hedrich, 1989; McAinsh et al.,
1990). ABA triggers cytosolic calcium ([Ca2+]cyt) increases (left panel; McAinsh et al., 1990). [Ca2+]cyt elevations activate two different types of anion channels: Slow-activating sustained (S-type; Schroeder and Hagiwara, 1989 ) and rapid transient (R-type; Hedrich et al., 1990) anion channels. Both mediate anion release from
guard cells, causing depolarization (left panel). This change in membrane potential deactivates inward-rectifying K+ (K+in)
channels and activates outward-rectifying K+ (K+out) channels (Schroeder et al., 1987), resulting in K+ efflux
from guard cells (left panel). In addition, ABA causes an alkalization of the guard cell cytosol (Blatt
and Armstrong, 1993) which directly enhances K+out channel activity (Blatt and Armstrong, 1993; Ilan et al., 1994; Miedema and Assmann, 1996) and down-regulates
the transient R-type anion channels (Schulz-Lessdorf et al., 1996). The sustained efflux of both anions and K+ from guard cells via
anion and K+out channels contributes to loss of guard cell turgor, which leads to stomatal closing (left panel). As vacuoles can take up over 90% of the guard cell’s volume, over 90% of the ions exported from the cell during stomatal closing must first be transported from
vacuoles into the cytosol (MacRobbie, 1998; MacRobbie, 1995). [Ca2+]cyt elevation activates vacuolar K+ (VK) channels proposed to provide a pathway for Ca2+-induced K+ release from the vacuole (Ward and Schroeder, 1994). At resting [Ca2+]cyt, K+ efflux from guard
cell vacuoles can be mediated by fast vacuolar (FV) channels, allowing for versatile vacuolar K+ efflux pathways (Allen and Sanders, 1996). The pathways for anion release from vacuoles remain elusive.
Stomatal opening is driven by plasma membrane proton-extruding H+-ATPases. H+-ATPases
can drive K+ uptake via K+in channels (right panel; Kwak et al., 2001). Cytosolic [Ca2+] elevations in guard cells down-regulate both K+in channels (Schroeder and Hagiwara, 1989) and plasma membrane H+-ATPases
(Kinoshita et al., 1995), providing a mechanistic basis for ABA and Ca2+ inhibition of K+ uptake during stomatal opening (right panel).
|